EMR to eSource: Why research sites don’t use EMR as the source data collection tool

73% of sites reported using paper as the primary means of data collection
In a 2018 CRIO site survey with over 100 respondents, sites reported that they use their Electronic Medical Record (EMR) primarily for patient recruitment purposes, but not for source data collection.
62% of site respondents reported that they use the EMR as the primary tool for patient recruitment, but only 13% cited the EMR as the primary tool for source data collection. Instead, 73% of site respondents reported using paper charts to collect source data. See chart below.
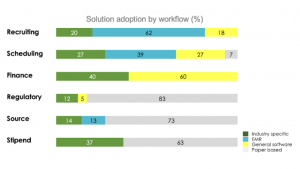
Notably, for source data, 14% of sites reported using an industry-specific electronic source (eSource) tool. Since this survey was taken, many more sites have adopted eSource, especially during the pandemic period when remote monitoring became commonplace. Today, CRIO estimates that as many as 20% of the research sites in the U.S. are using eSource.
EMR’s aren’t built to support clinical research the same way as eSource
This may be surprising to many, but it’s important to understand that an EMR is not designed to support the needs of a clinical trial. The EMR’s primary purpose is to give providers a flexible tool to document patient encounters and generate appropriate billing for insurance companies. EMR systems utilize standardized templates optimized for care delivery, not research.
By contrast, clinical trials have very specific and lengthy data requirements that vary with each protocol. To comply with study protocols, research staff have to schedule visits in a prescribed order, with limited variance against target dates; collect detailed, structured data; and follow rigidly prescribed workflows that dictate everything from how the vitals are to be taken (sitting vs. supine, right arm vs. left arm, etc.) to how the IP is administered.
It’s extremely difficult for most research personnel to configure the EMR visit schedules and data templates to meet the complex needs of a particular study. No health care institution could justify expanding the IT resources required to reconfigure the EMR to accommodate 5-10 study participants when they are managing hundreds of thousands of patient lives on the care side.
Care data and research data are different
Furthermore, the types of data used in the care and in research are of limited value to the other. On the care side, multiple providers need to access it, and each provider makes incremental contributions to the patient’s medical history. Over time, the data becomes large, messy, and outdated. Medical conditions, for example, often contain conditions that have self-resolved; hypotheses that did not bear out; or differential diagnoses for the same underlying condition.
Research requires precision. Study sponsors rely on the Principal Investigator to document the actual medical conditions of the patients, not past conditions or provisional hypotheses, and the actual medications the patient is taking, not just those that were prescribed but not taken. In addition, research protocols require documentation of study-specific data, such as pill counts or study-specific compliance, that are of value to the study sponsor but not to other care providers.
Today’s leading sites are transitioning from paper (or EMR) to eSource
For this reason, most research staff collect their clinical trial data outside of the EMR. Historically, this has been done in Word documents, using the site’s source templates. These documents are stored in patient-specific binders and reviewed by the CRA during on-site visits. Medical records, as needed, are printed and annotated, then inserted into the binder. Experienced CRA’s are well familiar with images like these from a research site:

This is where eSource comes in. eSource gives sites a tool they can use to configure study-specific visit schedules and procedures. It eliminates the use of paper, builds in quality checks at the point of data capture, and enables true remote monitoring – where data can be reviewed and queries issued in real-time as the visits are completed.
To date, over 900 sites in 14 countries use CRIO eSource for their clinical trial data capture needs. Many, if not most, still use the EMR – but mainly as a source of potential patients, and as an input to the clinical trial data (medical history, medications, adverse events). They will notate in the EMR the fact of the patient’s participation in the trial; the nature of the trial; and critical milestones, such as randomization or IP administration. But none of them use the EMR as their primary source data collection tool – in fact, several of CRIO’s clients tried and failed with EMR before adopting CRIO eSource.
eSource advances clinical research
From a sponsor’s perspective, eSource sites are the high-performing sites they should want in their studies. These sites have invested their own funds into automating their processes and building quality safeguards at the point of data capture. With CRIO’s eSource tool, these sites can easily provision CRA’s for remote monitoring. And with the pending launch of CRIO’s EMR integration, these sites will unlock the ability to pull in the patient’s medical charts digitally and have the PI review, annotate and approve the appropriate records for eSource, instead of having to obtain them in paper format and upload them into CRIO after annotation.
With better quality touch-points embedded throughout their processes, CRIO’s eSource-enabled sites are able to reduce their protocol deviations by up to 50% and improve data quality by 70%. This enhances PI oversight, promotes patient safety, and frees up the sites to focus on patient recruitment and retention, not clerical work.
Schedule a demo today to unlock the transformative benefits of CRIO eSource/ EDC.




