Ask Me Anything – Hitting the Target with Good Study Design
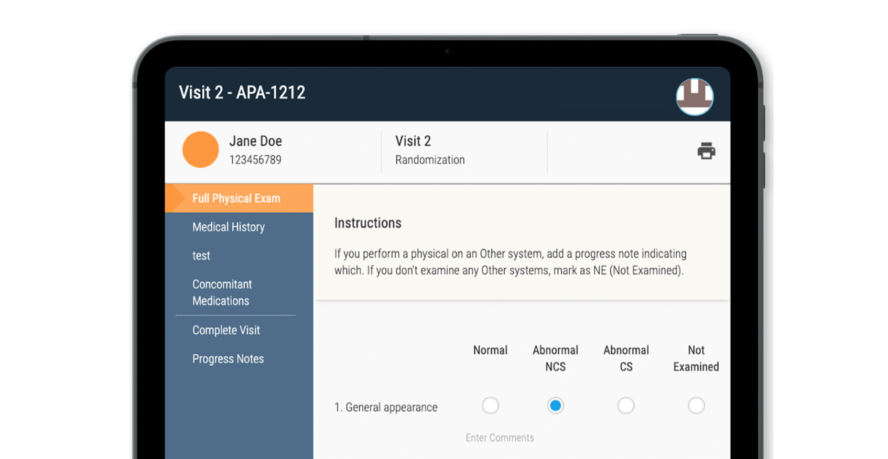
Mary Brenner, MSN MArch RN, is CRIO’s Study Design Manager and brings deep-rooted expertise as a registered nurse to CRIO’s in-house study design team. In this quick Q&A, Mary shares the benefits of good study design for sponsors and sites, the downstream effects of standardized study designs, and gives tips for designing it on your...